Schützenmeister Group Research
Rubrolides and Butenolides
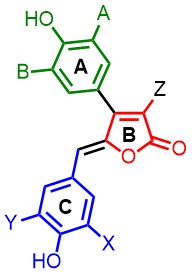
Rubrolides and Butenolides
Rubrolides are natural products, which were isolated from marine organism (e.g. ritterella rubra). The group is interested in synthesizing the natural products and analogues with high step- and atom-economy. The compounds are tested for antiviral and antibacterial activities.
For further information please visit:
- J. de Vries, M. Assmann, J. Janneschütz, J. Krauß, M. Gudzuhn, S. Stanelle-Bertram, G. Gabriel, W. Streit, N. Schützenmeister, Synthesis of Natural Rubrolides I, K, L, M, O and Analogues, Eur. J. Org. Chem. 2021, 2021, 4195-4200.
- M. Schacht, G. J. Boehlich, J. de Vries, S. Bertram, G. Gabriel, P. Zimmermann, P. Heisig, N. Schützenmeister, Protecting-Group-Free Total Syntheses of Rubrolide R and S, Eur. J. Org. Chem. 2017, 2017, 1745-1748.
- M. Schacht, D. Mohammadi, N. Schützenmeister, Pd-catalyzed Synthesis of γ-Ketoester as Key Intermediates for the Synthesis of γ-Hydroxybutenolides, Eur. J. Org. Chem. 2019, 2019, 2587-2591.
- M. Gudzuhn, I. Alio, R. Moll, J. de Vries, J. Boehlich, M. Assmann, J. Janneschütz, N. Schützenmeister, A. Himmelbach, A. Poehlein, R. Daniel, W. R. Streit, Molecular insight into gene response of diorcinols and rubrolide-treated biofilms of the emerging pathogen Stenotrophomonas maltophilia, Microbiol. Spectr. 2022, 10, e02582-21.
Diorcinols
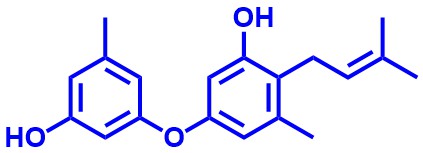
Diorcinols
The members of the Diorcinol family are natural products with a central diaryl ether motif. Some of the synthesized natural products of this family showed anti-MRSA and antibiofilm activity.
For further information please visit:
- G. J. Boehlich, J. de Vries, O. Geismar, M. Gudzuhn, W. R. Streit, S. G. Wicha, N. Schützenmeister, Total Synthesis of Anti-MRSA Active Diorcinols and Analogues, Chem. Eur. J. 2020, 26, 9846-9850.
- M. Gudzuhn, I. Alio, R. Moll, J. de Vries, J. Boehlich, M. Assmann, J. Janneschütz, N. Schützenmeister, A. Himmelbach, A. Poehlein, R. Daniel, W. R. Streit, Molecular insight into gene response of diorcinols and rubrolide-treated biofilms of the emerging pathogen Stenotrophomonas maltophilia, Microbiol. Spectr. 2022, 10, e02582-21.
C-Glycosidic Compounds and Scleropentasides
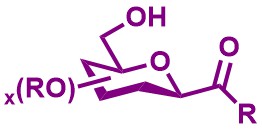
C-Glycosidic Compounds and Scleropentasides
Scleropentasides are naturally abundant C-acyl glycosidic compounds. The group developed a highly efficient and selective one-pot reaction to built up unprotected C-glycosidic compounds.
For further information please visit:
-
- G. J. Boehlich, H. Sterzel, J. Rehbein, N. Schützenmeister, Efficient Copper-Catalyzed Highly Stereoselective Synthesis of Unprotected C-Acyl Manno-, Rhamno- and Lyxopyranosides, Chem. Eur. J. 2022, 28, e202202619.
- G. J. Boehlich, H. Sterzel, J. Rehbein, N. Schützenmeister, Efficient Copper-Catalyzed Highly Stereoselective Synthesis of Unprotected C-Acyl Manno-, Rhamno- and Lyxopyranosides, Chem. Eur. J. 2022, 28, e202202619.
- G. J. Boehlich, N. Schützenmeister, β-Selektive C-Glycosylierung und ihre Anwendung in der Synthese von Scleropentasid A, Angew. Chem. 2019, 131, 5164-5167; β-Selective C-Glycosylation and its Application in the Synthesis of Scleropentaside A, Angew. Chem. Int. Ed. 2019, 131, 5110-5113.
- J. Boehlich, N. Schützenmeister, β-Selective One-Pot Synthesis of Acyl-C-Glycosides via Corey-Seebach Umpolung Reaction, SYNLETT 2019, 30, 1935-1939.

